Want to discuss your project or have any questions?
In close collaboration with you and tailored to your specific needs, we develop a wide range of high-throughput screening (HTS)-compatible cell-based assays. These include classical end-point assays, kinetic readouts and high-content analyses (HCA). All assay types can be developed to HTS standards, accommodating up to 384-well or 1,536-well microtiter plate formats.

Classical cell-based assays with end-point readouts remain the most popular choice for HTS. We specialise in a variety of assay types, including reporter gene assays, second messenger assays, protein degradation assays, protein–protein interaction assays, secretion assays and biomarker assays. Our advanced readout technologies include TR-FRET/HTRF, luminescence, fluorescence intensity and mass spectrometry. We utilise state-of-the-art multimode readers such as BMG Pherastar, PerkinElmer Viewlux and RapiFlex MS MALDI (Bruker) to enable precise and reliable results. Overview of assay types, readout technologies and multimode readers:
Cell-based assay types
Readout technologies
Multimode readers: BMG Pherastar, Perkin Elmer Viewlux, RapiFlex MS MALDI (Bruker)
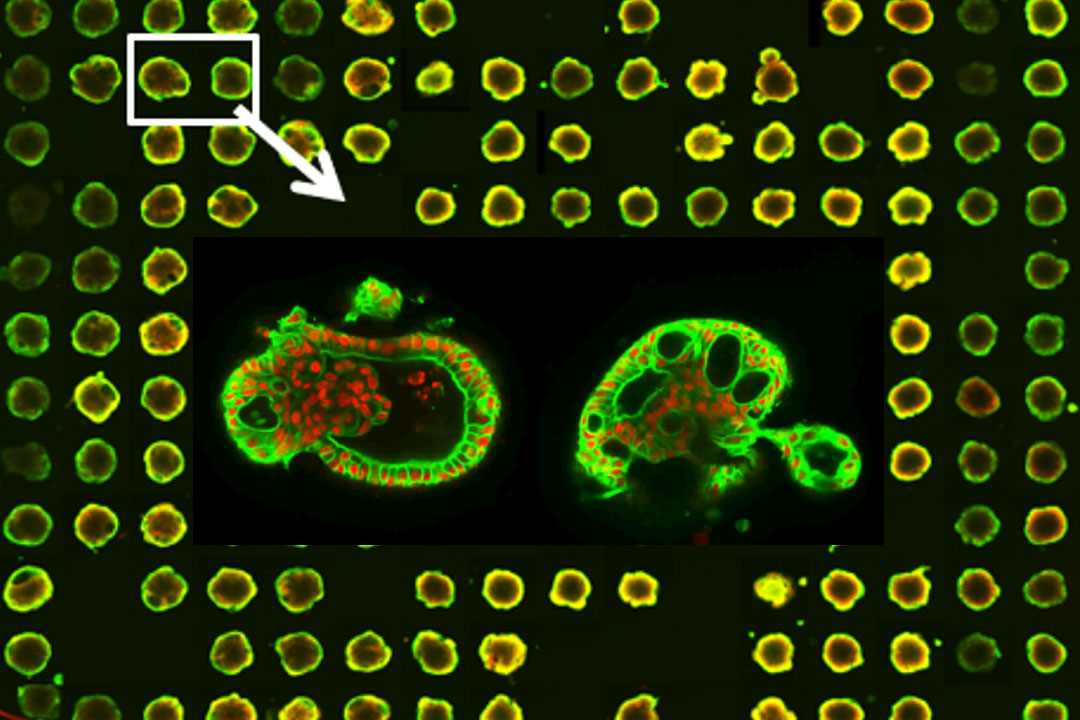
For challenging target classes, such as ion channels (e.g., potassium channels) or GPCRs, we offer high-resolution kinetic assays. We also analyse calcium dynamics in iPSC-derived cardiomyocytes or neurons. Our capabilities include full-deck library screens and regular compound testing. We use the industry-standard FLIPR platform, with one FLIPR Tetra and one FLIPR Penta, and employ 384- or 1,536-well pipettor heads for high-throughput applications. Additionally, we provide multidimensional time-lapse imaging via high-content analysis to better understand cell-drug response dynamics, including cell-cycle assays, autophagy assays, multi-target translocation assays, the determination of degradation kinetics or Ca2+ flux in sub-cellular compartments.
HCA is increasingly vital for the pharmaceutical industry to understand spatial and temporal phenotypic changes in cells. At Nuvisan, we consider HCA a crucial part of early drug discovery and have dedicated an entire platform to it. Our advanced high-content imaging and analysis capabilities have been successfully used to develop a wide range of assays and screen compound libraries of up to 4.5 million compounds.